Liming Li 10/Jul/2024
Follow me on LinkedIn:
https://www.linkedin.com/in/liming-li-81345a2a0/
The project was completed by me and three other undergraduate students when I was an undergraduate. The original version of this report is in Chinese, and it was not written by me. I translated it into English again for record.
1. Background
In keloid treatment, long-term drug therapy combined with pressure therapy is the most effective and cost-efficient method. However, drug therapy has side effects, and pressure therapy is limited to small areas. A fibrous drug carrier is proposed to control drug release and minimize side effects, particularly for corticosteroids.
2. Purpose
The project aims to develop a multi-layer composite dressing for controlled drug release to repair trauma and scars. It utilizes the drug clobetasol for its strong anti-inflammatory and anti-allergic effects, aiming to inhibit scar formation and proliferation. The sustained-release layer uses electrospinning to optimize drug release, while the overall dressing is breathable and antibacterial, promoting skin repair. Additionally, the project focuses on characterizing different rough structures of composite fiber membranes to assist various drugs in scar repair.
3. Experimental design
3.1 Preparation of Fibre Membrane
3.1.1 Preparation of Composite Fibre Membrane of Smooth Structure PCL + Triamcinolone acetonide
Inject PQ0, PQ5, PQ10, and PQ15 spinning solutions into stainless steel medical sterile syringes with different needle sizes. The needle of the syringe is connected to the positive pole of the high-voltage power supply through a metal clamp, and the aluminum foil is used as the fiber receiver and connected to the negative pole of the high-voltage power supply. Different nanofiber membranes of different mass concentrations of PCL spinning solution are collected, namely PQ0, PQ5, PQ10, and PQ15.
3.1.2 Preparation of PCL+Triamcinolone acetonide+SNC Composite Fiber Membrane with Serial Crystal Structure
A polycrystalline structure PCL + Triamcinolone acetonide + SNC composite fiber membrane was obtained by using the solution-induced crystallization method based on PQ10. Following the steps in 3.1.2, SNC, accounting for 5%, 10%, 15%, 20% of the solution mass, was separately added to PQ10 (3cm×3cm) in an amount of 500μL and then vacuum-dried at 37°C for 48 hours after the addition. The composite fiber membranes with crystalline structures prepared from PCL solutions with mass fractions of 5%, 10%, 15%, 20% are referred to as PQ10-S5, PQ10-S10, PQ10-S15, and PQ10-S20, respectively.
3.1.2 Preparation of PCL+Triamcinolone acetonide+SNC Composite Fiber Membrane with Bead String Structure
Prepare a mixed solution of PCL and SNC, with the mass concentration of SNC set at 5%, 10%, 15%, and 20% compared to PQ10, and an equal mass of triamcinolone acetonide added. The spinning solution is injected into a syringe with a 0.7 mm inner diameter needle, and is uniformly spun out at a constant rate (0.02 ml·min⁻¹) under the push of a micro syringe pump. The distance between the needle tip and the receiving plate is 15 cm, and the spinning voltage is 12 kV. The composite fiber membranes prepared from the spinning solution are PQ10-S5, PQ10-S10, PQ10-S15, and PQ10-S20, respectively.
3.2 Test Methods
3.2.1 Slow-release Effect Test
A series of sustained-release effect tests were conducted on the multi-layer composite functional dressings for scar repair preparation. Drug loading and release tests were performed on structures with different parameters to record the impact of drug release effect and to establish a pharmacokinetic model for the crystal structure, in order to find the appropriate release rate and drug action time.
3.2.2 Drug Delivery Method Testing and Selection
Compare the drug loading and drug release effects of two drug delivery methods and comprehensively compare and select the optimal solution. One is to mix the drug with nanofiber membrane, and the other is to attach the drug to the surface of the nanofiber membrane. Record and compare the drug loading and release effects of the two methods and comprehensively compare and select the optimal solution.
4. Final Parameter Selection:
4.1 Preparation Process and Parameter Selection for PCL Fiber Membrane
Spinning solution parameters: PCL concentration of 15%, solvent is a mixed solution of methanol and chloroform in a volume ratio of 3:1, the mixed solution is magnetically stirred at 60°C for 12 hours with a speed of 500r/min.
Spinning parameters: needle gauge of 21G, voltage of 20kV, spinning feed rate of 1ml/h, receiving distance of 20cm, maintaining environmental temperature at 22-25°C, and relative humidity at 30%-70%. After spinning, the fiber membrane is vacuum-dried at 37°C for 24 hours to remove residual solvent. Spinning time is 5-7 hours.
4.2 Induction Process and Parameter Selection of Fibrous Surface Polycrystalline Structure
Parameter selection – induction crystallization time: When the crystallization time reaches 5 minutes, a relatively complete and periodically arranged lamellar crystal structure can be formed on the surface of the fiber. Within a certain range as the crystallization time increases, the lamellae continue to grow, and the lamellar size and periodic distance gradually increase, forming a rough surface similar to collagen fibers. In the case of electrospun fiber raw materials being pure PCL, when the crystallization time is too long, the difficulty of forming a stable lamellar structure and morphology increases. Therefore, the parameter 30 minutes is chosen for moderate difficulty in preparation and a relatively suitable lamellar structure formation.
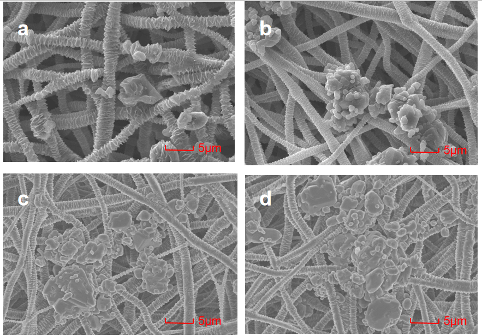
4.3 PCL Crystalline Fiber Membrane Drug Loading Process and Parameter Selection
The specific relevant content for this request, if necessary, delimited with characters: Fixed parameter selection — drug soaking time: Take several PCL electrospinning amorphous fiber membranes, and soak them in the soaking solution with a concentration of 20mg/ml for 10min, 30min, 60min, and 120min, respectively, according to the above drug-loading method. Take micrographs at a low magnification of 3k using the FlexSEM1000 scanning electron microscope, as shown in Fig 1. It can be seen that when the soaking time is less than 60min, the density of drug loading increases with the increase of soaking time, and the uniformity does not differ significantly (Fig1 a, b, c); when the soaking time exceeds 60min, the situation of drug loading will hardly change with the increase of soaking time (Fig1 c, d). Therefore, controlling the soaking time at 60min for subsequent experiments.
5. Test Results of Drug-Loaded Fibrous Membrane for Controlled Release
By using high performance liquid chromatography (HPLC), we obtained: The release amount of budesonide is calculated by determining the sample using high performance liquid chromatography and calculating the absorbance peak area. The chromatogram of the standard substance is shown in Fig 2, and the retention time of the characteristic peak of budesonide is around 5.347 minutes, and the peak area is linearly related to the drug content.

During the experiment period, the drug release amounts in the bar chart summaries (Fig. 3 and 4) were greatly influenced by multiple factors, resulting in significant differences in drug release amounts among the various sample films. Samples with a spherulitic structure are denoted as ‘c’ and those without a spherulitic structure are denoted as ‘f’. The drug soaking solution concentration is represented by a numerical value; for example, “c-40” represents “PCL spherulitic fiber membrane obtained by soaking in a 40mg/mL drug soaking solution”.
Drug Release Amount vs. Experiment Time: Within 24 hours, the drug release amount of group C film increased with the extension of time, and the growth rate and amplitude decreased with the extension of time. A significant increase in the release amount of the F group film was also observed. Within 5 days, the drug release amount of group C film continued to increase with time, but the increase was smaller than within 24 hours. It was almost impossible to observe an increase in the release amount of the F group film.
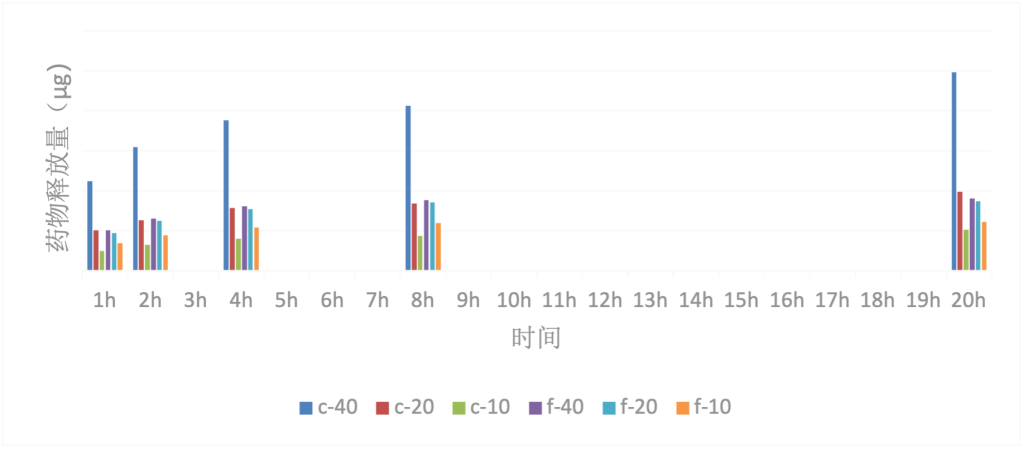


Drug release and drug loading of drug film:
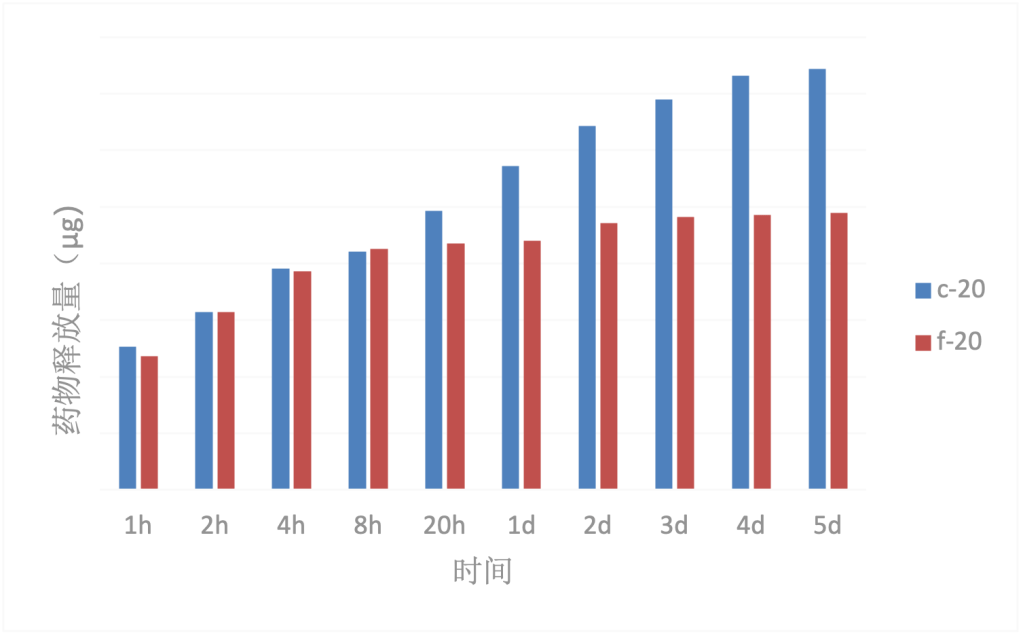
From Fig. 5, it can be seen that there is an approximately linear relationship between the drug loading of the fibrous crystalline membrane and the concentration of the immersion solution, but there is no such relationship between the drug loading of the non-fibrous crystalline membrane and the concentration of the immersion solution.
Drug Release and Fiber Structure: According to Fig. 6, under the same soaking liquid concentration conditions, the initial drug release amounts of both types of fibers are similar. However, the total drug release amount of the ribbon crystalline fiber membrane is greater than that of the non-ribbon crystalline fiber membrane, indicating that the drug loading capacity of the ribbon crystalline fiber membrane is superior to that of the non-ribbon crystalline fiber membrane. Additionally, the drug sustained release property of the ribbon crystalline fiber membrane is also significantly better than that of the non-ribbon crystalline fiber membrane. The total drug release amount of the ribbon crystalline fiber membrane continues to increase within 5 days, with the growth trend leveling off at 4-5 days. On the other hand, the drug release amount of the non-ribbon crystalline fiber membrane shows an increasing trend within 8 hours, and after 20 hours, the total release amount remains constant as the drug has been completely released.
In addition, as can be seen from Fig. 7 and 8, the drug release percentage curves of the drug films with the same fiber structure at different drug loading levels almost overlap. The trend of drug release from films with different drug loading levels is the same, indicating that the drug release percentage is not affected by the drug loading level.
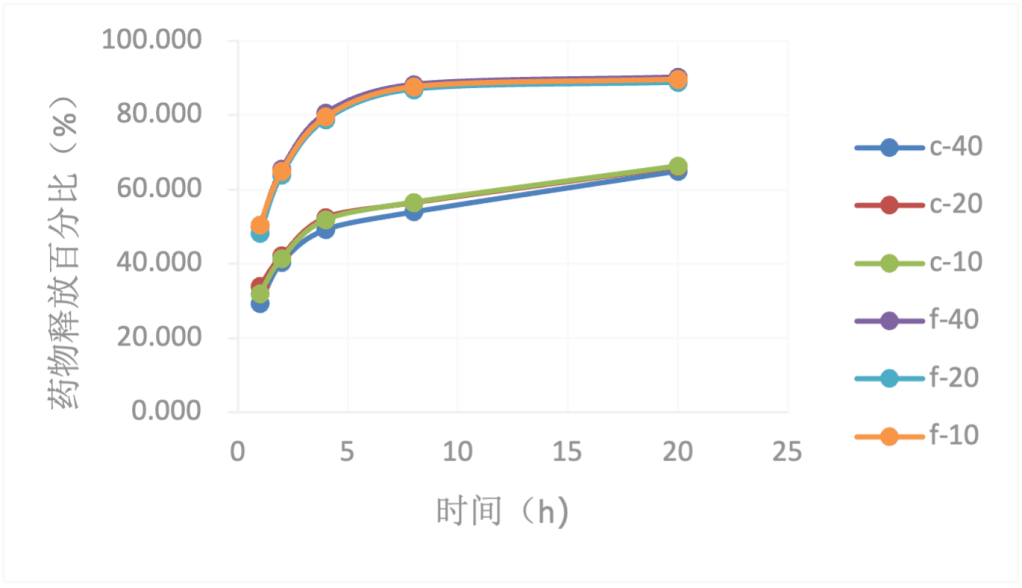

In summary, for beclomethasone, the electrospinning combined with drug-soaking method has very limited sustained release effect, but the introduction of spherulitic structure greatly improves the sustained release performance. Although it does not achieve linear sustained release, it extends the drug release period from within 1 day to 4-5 days. This drug release behavior is relatively stable and less affected by the drug loading amount, and the drug release can also be controlled by the drug loading amount.
Combining the physical and chemical properties testing and characterization in the second part of the samples, one possible reason for the above experimental results is that the PCL spherulitic fiber drug film, due to the surface modification of the fibers, allows deeper drug penetration, resulting in a longer release period. Another reason is that the drug attachment is multilayered: accumulation on the surface, aggregation at fiber intersections or in pores, and attachment to the fiber surface. Therefore, the drug release is also multilayered, and ultimately, these interactions collectively form the sustained release.
6. Summary and Outlook
Combining electrospinning technology with solution-induced crystallization, a quinacrine/PCL spherulite fiber drug sustained-release carrier was successfully prepared by inducing spherulite structure on the surface of PCL nanofibers.
Based on the results of microstructure testing, the sustained-release mechanism of the spherulite-structured PCL electrospun drug-loaded membrane may be (1) the spherulite structure occupies the space between the fibers, forcing the fiber interspace to increase, the porosity to become higher, and the drug to more easily penetrate with the solvent (or water), allowing the drug to not only stay on the fiber surface but also improve the hydrophilicity of the fiber membrane; (2) the affinity between the drug and the carrier contributes to the sustained-release effect; (3) the spherulite structure provides binding sites for drug attachment, allowing for the surface-adsorbed drug and the drug remaining in the pores to be released first, because the drugs attached to the spherulites and closely combined with the fibers are released later, thus forming sustained release; (4) in addition, the drug release behavior of the drug membrane is not significantly related to the drug loading, mainly related to the fiber membrane structure, etc., but based on the physical and chemical properties and drug release test results, the optimal drug-loading solution concentration is 20mg/mL. This indicates that the prepared PCL spherulite fiber drug membrane can achieve a certain degree of sustained drug release effect, and the drug release behavior of the carrier is relatively stable and the drug release can be adjusted through drug-loading parameters, preliminarily possessing the performance that a drug sustained-release carrier should have.
The research conducted in this experiment is limited due to objective and subjective abilities, and there are still many shortcomings. However, some experience has been accumulated. We believe that areas for improvement include: (1) further control of parameters during electrospinning to form fibers with diameters, fiber packing density, and orientation more suitable for the microscale electrospun fiber membrane of Curanid drugs; (2) in the process of crystal induction, some protective components can be added to PCL to prolong the crystallization time without causing preparation failure; (3) based on the drug release mechanism of crystal-induced PCL electrospun drug-loaded fiber membrane, achieve a sustained release equation.
Leave a comment